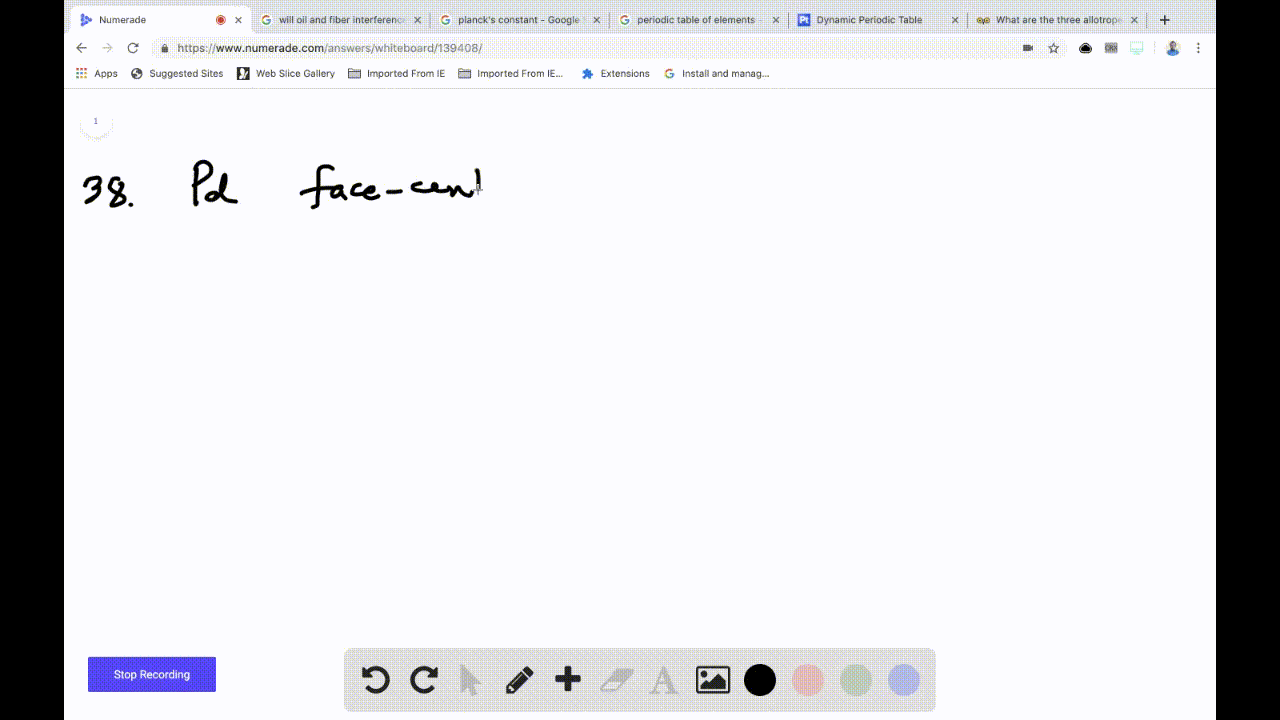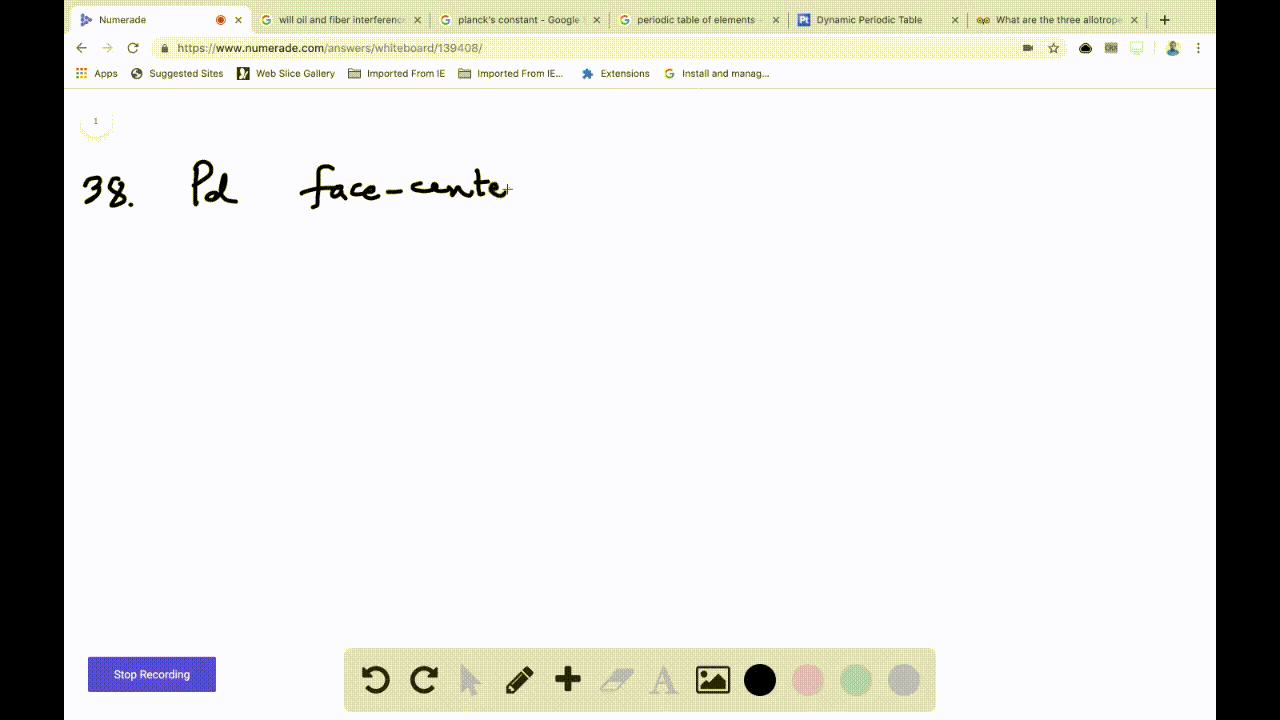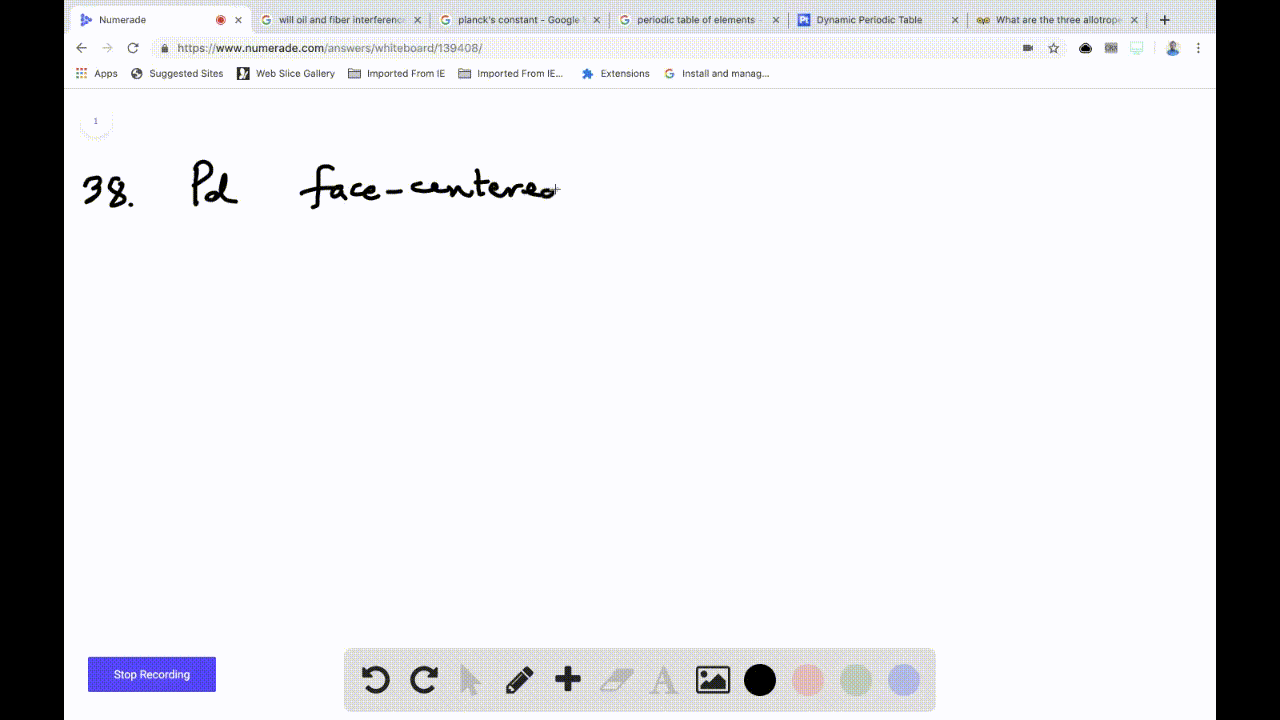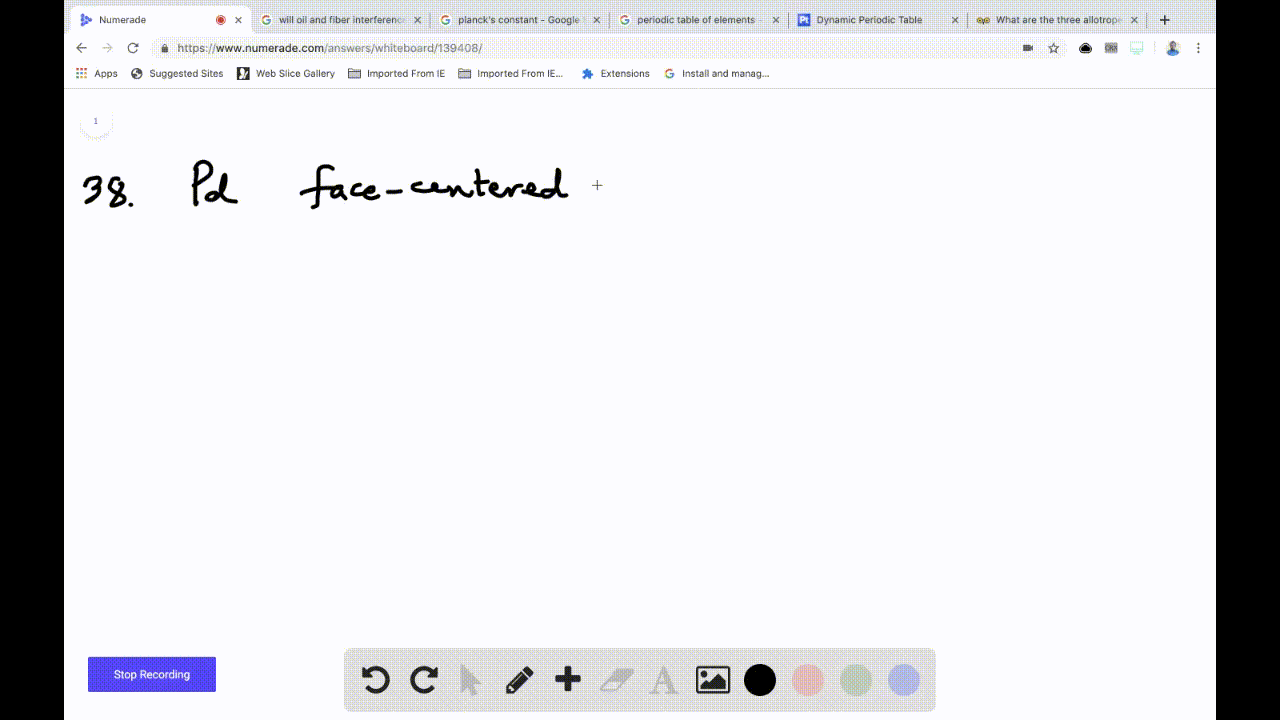
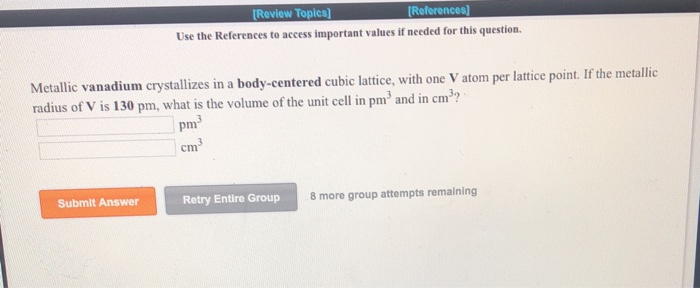
OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...

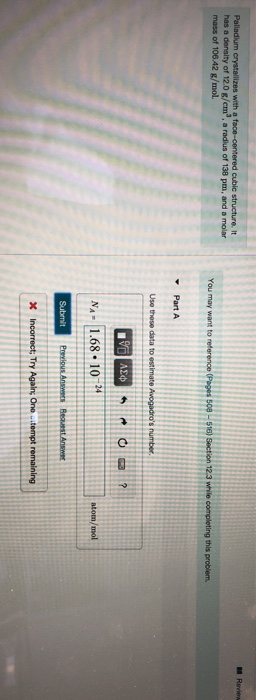
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's

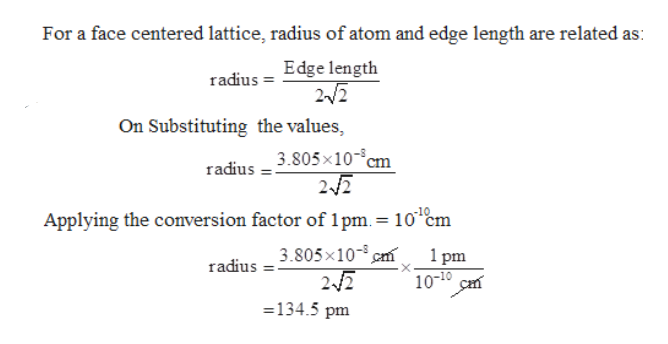
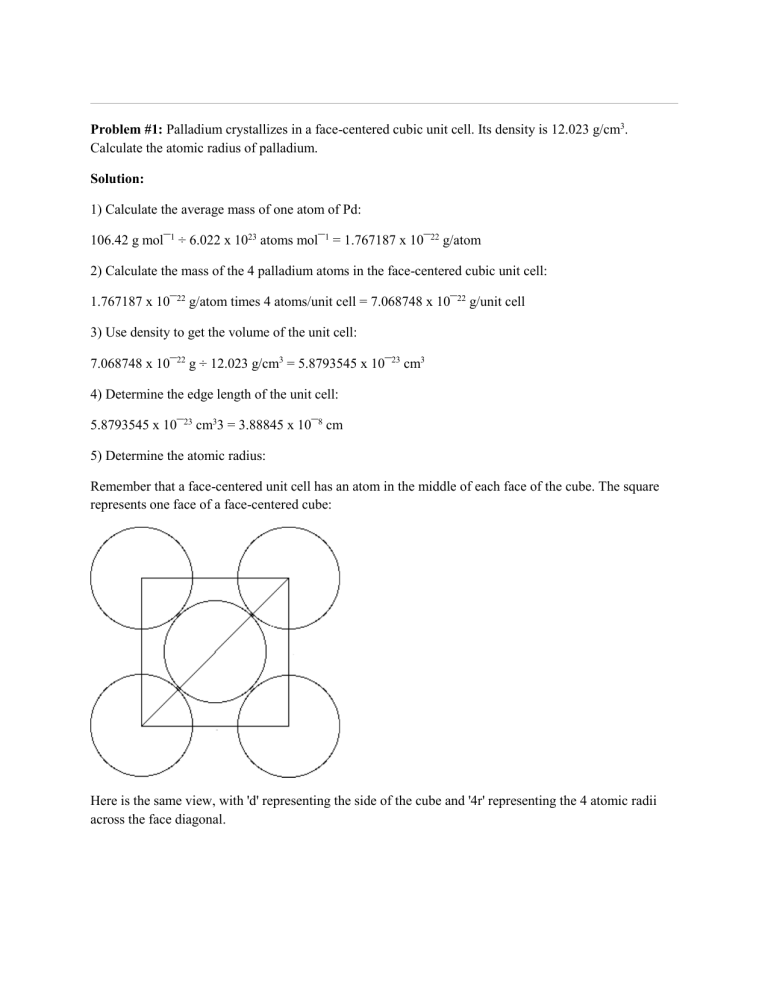
Problem #1: Palladium crystallizes in a face-centered cubic unit cell Its density is 12023 g/cm 3 Calculate the atomic radius of palladium | Course Hero

Document - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero

SOLVED:**30) Gallium crystallizes in primititre cubic Unit cell The length of the unit cell edge is 3.70 4 The radius ofa Ga atom is 4)7.40 B) 3.70C) 1.85D) 0.930 E) Insufficient data

Chapter 3 Homework - Chapter 3 Homework Textbook Problems 9 17 22 25 33 40 44 46 71 79 Additional problems and solutions Problem#1 Palladium | Course Hero

Problem.docx - Problem#1 Palladium crystallizes in a face-centered cubic unit cell Its density is 12.023 g\/cm3 Calculate the atomic radius of palladium | Course Hero
If the radius of palladium is 248 pm and the lattice type is body centered cubic, what is the - Sarthaks eConnect | Largest Online Education Community

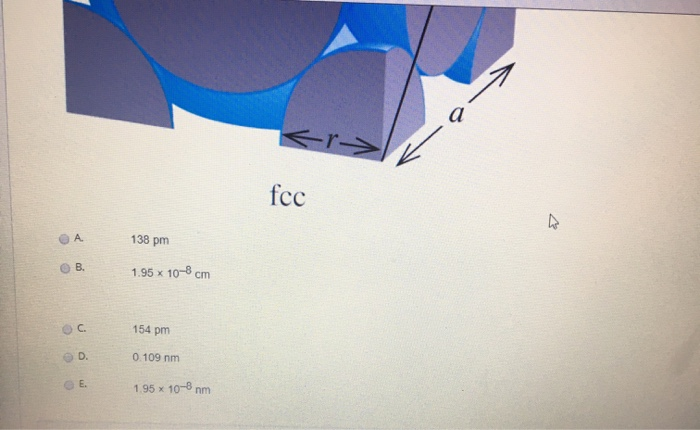
SOLVED:16. Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 glem'. What is the atomic radius of Pd? A. 138 pm B. 1.95 * 10 8 nm C.1.95 * 10-8 cm D. 154 pm E. 0.109 nm